Introduction
With increasing lifespans and improvement in the quality of life of elderly, joint replacements have started accounting for an increasing number of surgical orthopedic procedures.
In a landmark study done in the United States of America, it was concluded that one in every five adults suffers from arthritis.[1] The treatment for arthritis can be conservative or surgical depending on the severity of the case. However, this ratio puts into focus the magnitude of the problem and the dire need to come with more sustainable solutions for joint replacement therapy.
Evolution of biomaterials in Orthopedics
The last few decades have seen tremendous growth and improvement in the field of biomaterials with the evolution of 3 generations [2]. The first generation of bioinert materials, the second comprising of bioactive and biodegradable materials and the third consisting of materials with the ability to simulate the responses at a molecular level.
First Generation of Bone Implants
The initially used biomaterials were introduced keeping their physical, mechanical and chemical properties in mind. It was deemed essential to achieve a reasonable combination of physical properties similar to the tissue being replaced along with minimal toxic response in the host.
The concepts of stress shielding, foreign body reaction, osseointegration and osteoinduction were not deemed as necessities. These implants included the stainless steel and cobalt-chrome based alloys. In 1940s Titanium and its alloys also joined the bandwagon. NiTi appeared in the 1960s with shape memory.
The first generation also includes ceramics like alumina, zirconia and other porous ceramics and polymers like silicone rubber, acrylic resins, polyurethanes, polypropylene (PP) and polymethylmethacrylate (PMMA).These materials led to adsorption of a layer of proteins on their surface resulting in unspecific signals to the cellular environment.
Second Generation of Bone Implants
This includes bioactive biomaterials that led to the in vivo deposition of a layer of hydroxyl-Apatite (HA) at the material surface or promote certain specific regenerative cell responses.
It comprises of ceramics eg: bioactive glass, glass–ceramics and calcium phosphates, surface coated metals and biodegradable polymers such as polyglycolide, polylactide, polydioxanone, polycaprolactone, poly-hydroxybutyrate, poly-orthoester, chitosan, poly(2-hydroxyethyl-methacrylate), hyaluronic acid and other hydrogels.
Third Generation of Bone Implants
This generation combines the bioactive and biodegradable aspects eg: temporary three-dimensional porous structures that stimulate cells' invasion, attachment, and proliferation, as well as functionalized surfaces with peptide sequences that mimic the ECM components so as to trigger specific cell responses.Both natural and synthetic polymers have been used in the development of new three-dimensional scaffolds.
The synthetic biodegradable polymers enable a better control of their physicochemical properties and have clinically used with success. PLA, PGA, PCL, and PHB are the most widely studied polymers for bone tissue engineering purposes.
Current research is focused on three-dimensional ACP scaffolds, nanocrystalline structures, microspheres, hierarchically organized structures and tuned porosity microstructures.
Evolution and Advances in Metallic Bone Implants
Stainless Steel and Austenitic Stainless Steel
The primary advantage of this material is the low cost, ease of availability and processing. However, inadequate corrosion resistance and average mechanical properties of stainless steel gave way to the new austenitic stainless steel with high Cr content (over 20%), where Ni has been partially substituted by Mn and with a high N content (between 0.3 and 0.4%), is being used in the joint prosthesis. N stabilizes the austenitic phase and induces an increase in both the corrosion resistance and the mechanical properties (yield stress).
The austenitic compound has poor corrosion resistance. Modification includes the introduction of a Co–Cr–Mo alloy (ASTM F75, Vitallium) in hip prostheses to combat all these problems.
The austenitic compound has poor corrosion resistance. Modification includes the introduction of a Co–Cr–Mo alloy (ASTM F75, Vitallium) in hip prostheses to combat all these problems.
Titanium and Alloys
Commercially pure Titanium has excellent mechanical properties, corrosion resistance, fatigue–corrosion resistance, low density and relatively low modulus.Processing is not easy whether it is machining, forging or heat treating.Some studies have also pointed out V as a potential cytotoxic element 3. These problems have been addressed by Ti6Al7Nb and Ti5Al2.5Fe, with properties similar to Ti6Al4V but V-free, and TNZT alloys, based on the TiNb Ta Zr system to achieve minimum elastic moduli and excellent biocompatibility.
The Ti35Nb5Ta7Zr and its variant Ti35Nb5Ta7Zr0.4O show elastic moduli values as low as 55 and 66 GPa. This new generation of Ti alloys is at present under development and investigation, and not commercialized yet.
NiTi Alloys
These have the property of shape memory. They can exhibit an elastic modulus as low as 30 GPa in the martensitic state, while it ranges between 70 and 110 GPa in the austenitic phase.
 |
| Metallic Implants for Knee Replacement |
Some other advances in metallurgy include the introduction of:
- Nickel-free stainless steel, cobalt-chromium and titanium alloys
- New alloys like tantalum, niobium, zirconium, and magnesium
- Metallic implants with lower modulus
TECHNIQUES TO MAKE METAL BONE IMPLANTS BIOACTIVE
Surface Modifications to Bone Implants
• Electrophoretic deposition (Ducheyne et al. 1990),
• Plasma spraying (De Groot et al. 1990; Thull& Grant 2001),
• Radiofrequency or ionic ray sputtering (Cook et al. 1988),
• Laser ablation (Clèries 1999; Serra et al. 2001) or
• Hot isostatic pressure (Hero et al. 1994)
None of these methods produce covalent links with the substrate, and the majority are not cost-effective. Covalent chemical binding of polymers and biomolecules has been achieved through silanized titania surfaces, using amino- and carboxyl-directed immobilization mainly through glutaraldehyde chemistry, and photochemistry by ‘grafting to’ biomolecules with a photoactive group (Colloiud et al. 1993; Xiao et al. 1998, 2001).
Chemical Modifications to Bone Implants
• Thermochemical treatment developed by Kokubo et al. (1996),
• Chemical etching with hydrogen peroxide containing small amounts of tin chloride (Ohtsuki et al. (1997)
• Dipping the material in a sol-gel solution previously prepared at 0°C, followed by a thermal treatment at 500°C (Li & de Groot 1993)
• Attachment of self-assembled monolayers with a functional group at their end, which is able to induce the nucleation of a CaP (Campbell et al. 1996; Tanashi& Matsuda 1997; Wheeler et al. 1997).
Mainly developed for CP Ti and Ti alloys to obtain apatite or other CaP material layers on metallic surfaces, creating a direct chemical link between the substrate and the coating.
Evolution and Advances in Ceramic Bone Implants
 |
| Knee Replacement Implants |
In order to promote bone ingrowth and stabilization of prosthesis, highly porous ceramics have been developed. However, mechanical requirements will determine the porous behavior for low loaded or unloaded bearing applications.
NON-OXIDE CERAMICS LIKE SILICON NITRIDE AND SILICON CARBIDE
This category comprises of Silicon carbide and Silicon nitride. They are glass-ceramic composites and have a good corrosion, fracture and creep resistance along with improved ductility and hardness.
These materials also have excellent cytocompatibility in vitro. Super oxidation of these compounds leads to oxide layer formation which chips off with time leading to significant wear. Ongoing studies with improvement in their microstructure will help in making them more viable.
These materials also have excellent cytocompatibility in vitro. Super oxidation of these compounds leads to oxide layer formation which chips off with time leading to significant wear. Ongoing studies with improvement in their microstructure will help in making them more viable.
Evolution and Advances in Polymers of Bone Implants
Despite its extensive use, PMMA bone cement is associated with various drawbacks such as residual monomer, an exothermic setting reaction which may lead to thermal necrosis of the surrounding bone, the shrinkage during polymerization producing gaps, the difference in stiffness between the prosthesis and the bone inducing overstress or overstrain resulting in fractures in the cement.
To improve the properties the ceramic radio pacifier particles are being replaced by opacifiers based on organic iodine compounds. (Artola et al. 2003).
Advances in handling techniques such as monomer cooling, vacuum mixing and injecting devices have led to improvement in microstructure and mechanical properties. In 2003 and 2004 Phillips and Bono and Garfin have used acrylic cement in vertebroplasty and kyphoplasty with success, finding new applications for them.
For osteosynthesis of plates and screws, polymer matrix composite materials reinforced with ceramic particles or fibers were developed. The main strategies consisted of reinforcing polymeric matrices of polyetheretherketone, PE or polysulphone (PS) mainly with carbon or glass fibers (Evans & Gregson 1998; Ramakrishna et al. 2001). Carbon fiber reinforced composites allow lower rigidity in comparison with metallic biomaterials, which is closer to cortical bone (Lewandowska-Szumiel et al. 1999). Self- reinforcing processes have also been used for enhancing the mechanical strength of bioabsorbable polymeric implants.
Bioabsorbable implants have also been used for spinal surgery. Some applications such as interbody cages elaborated with PLA for specific spinal fusion applications, bioabsorbable ramp type interbody spacers for posterior lumbar interbody fusion procedures and the use of PLA screws for anterior cervical decompression and fusion processes have been successfully reported.
HYDROGELS Use in Bone Implants
Hydrogels are novel polymers that gained more popularity in recent years. As an example of degradable water containing substances they can be injectable and of different water contents. They can consist of Poly-(ethylene glycol) or gelatin.
They are used experimentally and clinically as biomaterials for the controlled release of bone regeneration activity enhancing substances like Transforming Growth factor (TGF)-beta 1, Insulin-like growth factor (IGF)-1 and bone morphogenetic protein-2. They can also be used as scaffolds and carriers for osteoprogenitor and other cells like chondrocytes, fibroblasts, and mesenchymal stromal cells.
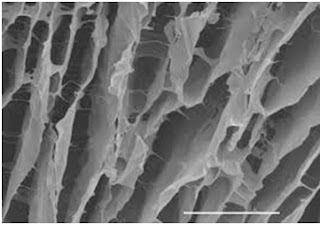 |
| Hydrogel Scaffold: Electron Microscopic View |
Advances in Design and Use of 3-D Technology in Bone Implants
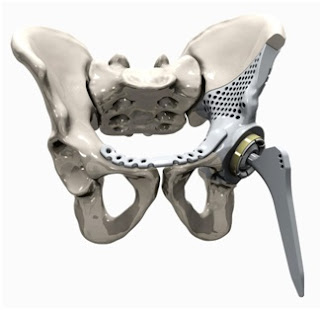 |
| 3D Printing of Hip Joint Replacement Implant |
These implants ensure better implant- bone match, better implant positioning, and alignment with other joint components, thus increasing the longevity of the implant. In implants with highly cross-linked polyethylene, Vitamin E incorporation is being discussed as it acts as an antioxidant and increases the life of the implant.
Advances in Orthobiologics and Impact on Bone Implants
These are calcium salt bone fillers containing demineralized bone matrix with bone growth stimulators. These help in regeneration of bone by means of osteoconductive, osteoinductive and osteogenic actions. With advances in tissue engineering and development of clinically viable scaffolds and ortho-biologic, alloplastic implants may become a thing of the past.
Conclusion
In spite of the great deal of research in the field of orthopedic implantology and joint replacement material, metallic implants rule the industry. The primary reason for this can be ascribed to their superior mechanical properties, like strength. Recent advances in metallurgy have helped in significant improvement in these properties while a reduction in the adverse ones.
Tissue engineering and stem cell therapies offering de novo generation and regeneration of implants seems to be a promising option. However, the issues of ethical clearance and technical difficulties associated with stem cell therapy increasingly shout out to improvement in alloplastic materials used in joint replacement or bone implants.
While a wide variety of seemingly biocompatible implants have been desired over the last century, the demand for the perfect implant material and design is yet to be fulfilled.
References
1. Helmick C, Felson D, Lawrence R, Gabriel S. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States, Arthritis Rheum. 2008 ; 58:15-25.
2. Hench L, Polak J. Third-generation biomedical materials. Science. 2002;295:1014–1017.
3. (Friberg et al. 1979; Steinmann 1985; Thompson & Puleo 1996; Daley et al. 2004).

No comments:
Post a Comment
We are happy that you want to comment, please note that your comment will be reviewed first before it is published.
If you like the article! You can share it with your friends and colleagues by pressing at social media buttons provided to the left of the page.
NO word verification or sign up is required!