Diabetes Disorder
Diabetes mellitus is a chronic metabolic disorder characterized by high blood sugar levels, which result from defects in insulin secretion or action or both. Normally, blood glucose levels are tightly controlled by insulin, a hormone produced by the pancreas.[1] Diabetes can be either type 1 or type 2.
Type 1 diabetes is also called as insulin dependent diabetes mellitus (IDDM) or juvenile onset diabetes mellitus. In type 1, the pancreas undergoes an autoimmune attack by the body itself and is rendered incapable of making insulin. Type 2 diabetes is referred as non-insulin dependent diabetes mellitus (NIDDM) or adult onset diabetes mellitus (AODM).
In type 2 diabetes, the patient can still produce insulin but do so relatively inadequately for their body's needs.[2] Complications of diabetes may be acute or chronic. An acute complication of type 1 diabetes involves diabetic ketoacidosis (DKA) and type 2 involves hyperosmolar coma.
Chronic complications are related to blood vessels and are classified into Microvascular disease or Macrovascular disease. The microvascular disease involves eyes, kidneys, and nerves causing blindness, kidney failure, and nerve damage. Diabetes accelerates hardening and narrowing of the arteries (atherosclerosis), leading to strokes, coronary heart disease, and other large blood vessel diseases. These are referred to as macrovascular diseases.[3] Chronic low-grade inflammation, which is present in both type-1 and type-2 diabetes, contributes to the pathogenesis of insulin resistance.
It is due to the accumulation of activated innate immune cells in metabolic tissues which results in a release of inflammatory mediators, to promote systemic insulin resistance and beta-cell damage.[4] Mechanism of insulin release in normal pancreatic beta cells - insulin production is more or less constant within the beta cells. Its release is triggered by food, chiefly food containing absorbable glucose. Insulin is the principal hormone that regulates the uptake of glucose from the blood into most cells of the body, especially liver, muscle, and adipose tissue.
Therefore, deficiency of insulin or the insensitivity of its receptors plays a central role in all forms of diabetes mellitus[5]. Insulin is a peptide hormone, therefore, destroyed by gastric acid if taken orally. Insulin can be administered subcutaneously via various methods such as vial and syringe, insulin pen and continuous subcutaneous insulin infusion[6].
Various forms and various routes of insulin have been found till now. But everyone has their own advantages and disadvantages. A novel pre-termed drug has also been found to treat diabetes. Insulin pen devices have several advantages over the traditional vial-and-syringe method of insulin delivery, including improved patient satisfaction and adherence, greater ease of use, superior accuracy for delivering small doses of insulin, greater social acceptability, and less reported injection pain.[7]
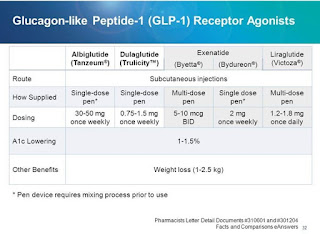
Introducing newer insulin analogue treatment in various forms have been increased nowadays. Glucagon-like peptide-1 (GLP-1) receptor agonists provide beneficial effects, including increased insulin biosynthesis and glucose-dependent insulin secretion, suppressed glucagon secretion, slowing of gastric emptying, and reduced food intake[8].
Liraglutide, lixisenatide, and exenatide are GLP-1 receptor agonists approved clinically for the treatment of type 2 diabetes. Dulaglutide is a long-acting GLP-1 receptor agonist recently approved in the United States and the European Union as a once-weekly s/c injection for the treatment ofT2D [9].
 |
| Deborah Sturpene Drug Update |
Various Insulin Delivery Methods [10]
Vial & Syringes
The intravenous route was the first parenteral route for drug delivery reported through syringes and needles in the late 17th century, and the subcutaneous route of drug delivery was established in the early 19th century. In 1924, 2 years after the discovery of insulin, Becton, Dickinson, and Company (BD) made a syringe specifically designed for the insulin injection.[9] Initial syringes were made of glass and/or metals, which were reusable and required boiling after each use to sterilize. To reduce the incidence of needle associated infections, disposable syringes were developed.
Insulin Pens
The first insulin pen was manufactured by Novo Nordisk in 1985. insulin pens are reusable, more accurate and equipped with safety features such as audible clicks with each dose to improve accuracy and reduce the chances of human errors.
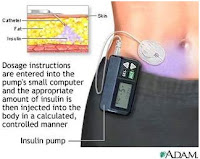
Continuous Subcutaneous Insulin Infusion
The first commercial insulin pump was introduced in 1979 in the USA. The current generation of insulin pumps is more patient-friendly as a result of smaller size and smart features such as built-in-dose calculators and alarms.
Sensor-Augmented Pump Therapy
 |
| Insulin Pump |
Sensor-Augmented Pump With Low Glucose Suspend Or Threshold Suspend Pump
This is the first step in making an artificial pancreas (closed-loop system) to suspend insulin delivery once CGM glucose is at a low threshold (often 70 or 60 mg/dl) to reduce nocturnal hypoglycemia.The threshold suspends (TS) system suspends the delivery of insulin for up to 2 h if a patient does not take action with a low glucose alarm. This feature is designed to reduce the severity and duration of hypoglycemia, although it will not prevent hypoglycemia.
Inhaled Insulin
This route includes a vast and well perfused absorptive surface, absence of certain peptidases that are present in the gastrointestinal (GI) tract that breaks down insulin, and the ability to bypass the “first pass metabolism.
Oral Insulin
Certain oral insulin preparations such as Capsulin, ORMD-0801, IN-105, oral hepatic directed vesicles and Eligen completed phase 1 and phase 2 trials with promising results
Nasal Insulin
Nasulin™ (CPEX pharmaceuticals) and nasal insulin by Nastech Pharmaceutical Company Inc. Both insulin preparations have bioavailability of about 15-25% with the onset of action ~10-20 min
Transdermal Insulin
Transfersulin is the insulin encapsulated in Transferosome, an elastic, flexible vesicle which squeezes itself to deliver drugs through skin pores. These are still evolving and their long-term utility, safety, and usefulness are not known at present.
Intraportal Insulin
Direct delivery of insulin in the portal vein mimics the high portal insulin concentration.But it is invasive, may be associated with subcutaneous infections, cannula blockage, higher cost, portal-vein thrombosis and peritoneal infection.
Comparison Between Insulin And Non-Insulin Devices [11]
Insulin-Delivery Pens
Insulin-delivery pens eliminate the need to draw up insulin, which allows more convenient administration compared with syringes. An insulin pen looks like a large fountain pen. The pen is prefilled with insulin, and the only preparation required is attaching the needle. There are two basic types of pens: disposable and reusable. The following instructions for use are common among devices
 |
| Insulin Pens |
1. Remove the pen cap and attach the pen needle by twisting it onto the rubber stopper of the cartridge.
2. Perform an “air shot” (also called priming the pen or a safety test, depending on the manufacturer of the pen that the patient uses) to remove air bubbles.
a. Dial a test dose of 2 units.
b. Hold the pen with the needle pointing up and gently tap the cartridge so that air bubbles rise up to the needle.
c. Press the injection button all the way to 0 and check to see that insulin comes out of the needle; then3. Dial your dose and, while keeping the pen straight down
or at a 90-degree angle, insert the needle into the skin.
4. Press the injection button all the way down with your thumb.
5. Hold for five to 10 seconds to ensure full-dose delivery.
6. Release the button; remove the needle from the skin; twist off the pen needle to discard it, and recap the pen.
Non-insulin Devices
1. Remove the pen cap and attach the pen needle by twisting it onto the rubber stopper on the cartridge.
2. Perform a one-time-only pen setup. This is required only the first time a new pen is used.
a. Pull the dose knob out and dial the dose.
 |
| Trucility Injection Pen |
3. Dial the dose and, while keeping the pen straight down (at a 90-degree angle), insert the needle into the skin.
4. Press the injection button all the way down with the thumb.
5. Hold the button for five to 10 seconds to ensure full-dose delivery 6. Release the button; remove the needle from the skin; twist off the pen needle to discard it, and recap the pen.
Entry Of Non-Insulin Devices[13]
Dulaglutide is a novel glucagon-like peptide-1 agonist (GLP-1) biologic drug consisting of a dipeptidyl peptidase-IV-protected GLP-1 analog covalently linked to a human IgG4-Fc heavy chain by a small peptide linker. Dulaglutide is indicated in the treatment of type 2 diabetes and can be used once a week.
Protein structure classification of dulaglutide:
 |
| The Dulaglutide Molecule |
Color code in B is representative of region color in A.
GLP-1: glucagon-like peptide-1; IgG: immunoglobulin gamma; Fab: fragment antigen binding; Fc: fragment crystallizable
Indication Of Dulaglutide
Indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Pharmacodynamics Of Dulaglutide
Dulaglutide activates human glucagon-like peptide-1 receptors, thus increasing intracellular cyclic AMP in beta cells. This, in turn, increases glucose-dependent insulin release. Dulaglutide also reduces glucagon secretion and slows gastric emptying.
Mechanism of Action Of Dulaglutide
Dulaglutide is a human GLP-1 receptor agonist with 90% amino acid sequence homology to endogenous human GLP-1 (7-37). Dulaglutide activates the GLP-1 receptor, a membrane-bound cell-surface receptor coupled to adenylyl cyclase in pancreatic beta cells.
Dulaglutide increases intracellular cyclic AMP (cAMP) in beta cells leading to glucose-dependent insulin release. Dulaglutide also decreases glucagon secretion and slows gastric emptying.
Absorption Of Dulaglutide
Maximum plasma concentration (Cmax) is achieved in 24–72 h (median 48 h) after a subcutaneous injection administered at a steady state.
After subcutaneous administration of 0.75 mg and 1.5 mg to a steady state were approximately 19.2 L (range 14.3 to 26.4 L) and 17.4 L (range 9.3 to 33 L), respectively.
Metabolism Of Dulaglutide
Dulaglutide is presumed to be degraded into its component amino acids by general protein catabolism pathways.
Half-life Of Dulaglutide
Approximately 5 days.
Clearance Of Dulaglutide
The mean apparent clearance at steady state of dulaglutide is approximately 0.111 L/h for the 0.75 mg dose, and 0.107 L/h for the 1.5 mg dose.
Case Studies Of Dulaglutide
Dulaglutide for type 2 diabetes
Dulaglutide-treated patients achieved the composite endpoint of an HbA1c <7.0% with no hypoglycemia, no severe hypoglycemia, and no weight gain significantly more than metformin, sitagliptin, exenatide BID or insulin glargine-treated patients. Dulaglutide consistently showed an early onset of glycemic control, lasting up to 104 weeks.[14]
Dose Findings Results of Once-weekly Dulaglutide
It is an adaptive, seamless, double-blind study comparing dulaglutide, a once-weekly glucagon-like peptide-1 (GLP-1)
a receptor agonist, with placebo at 26weeks and sitagliptin up to 104 weeks.As a result, the Bayesian algorithm allowed for an efficient exploration of a large number of doses and selected dulaglutide doses of 1.5 and 0.75mg for further investigation in this trial.[15]
1-Year Safety Study Of Dulaglutide In Japanese Patients
the safety and efficacy of 0.75 mg of dulaglutide, a once weekly, in Japanese patients with type 2 diabetes (T2D) on a single oral hypoglycemic agent (OHA). A significant increase was observed in combination with Thiazolidinedione, there were no significant changes in combination with Sulfonylureas, and significant reductions were observed in combination with Biguanides. Once weekly dulaglutide 0.75 mg in combination with a single OHA was overall well tolerated and improved glycemic control in Japanese patients with T2D.[16]
Safety And Side Effects
Safety
The once-weekly dulaglutide single-dose pen can be safely and effectively used for self-injection by patients with T2D who are naïve to self-injecting or injecting others. The primary and secondary objectives were met, with final injection success observed in 99.1% of patients (95% CI: 96.6% to 99.7%) and initial injection success observed in 97.2% (95% CI: 94.0% to 98.7%). Final self-injection success was 99.3% in patients aged <65 years and 98.6% in those aged ≥65 years, suggesting that age is not a factor in the successful use of the dulaglutide single-dose pen.[17]
Side Effects
In a 52-week study designed to quantitate C-cell mass and plasma calcitonin responses, rats received twice-weekly sc injections of dulaglutide 0 or 5 mg/kg. Dulaglutide increased focal C-cell hyperplasia; however, quantitative increases in C-cell mass did not occur. Consistent with the lack of morphometric changes in C-cell mass, dulaglutide did not affect the incidence of diffuse C-cell hyperplasia or basal or calcium-stimulated plasma calcitonin, suggesting that diffuse increases in C-cell mass did not occur during the initial 52 weeks of the rat carcinogenicity study.[18]
In risk of thyroid c-cell tumors -Dulaglutide is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2. Routine serum calcitonin or thyroid ultrasound monitoring is of uncertain value in patients treated with Dulaglutide.[13] Symptoms of overdose may include the following: nausea, vomiting, symptoms of hypoglycemia.
Dulaglutide injection may increase the risk that you will develop tumors of the thyroid gland, including medullary thyroid carcinoma (MTC; a type of thyroid cancer). Laboratory animals who were given dulaglutide developed tumors, but it is not known if this medication increases the risk of tumors in humans[19].
REFERENCES
1. Nicholas A.Boon, Nicki R.Colledge, Brain R.Walker and John A.Hunter: Davidson's principle and practice of medicine-20th edition.2006.p.805-847.
2. Rippe, James M. Manual of intensive care medicine (5th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. ISBN 9780781799928.,(2010). p. 549.
3. RCG Rusell, Norman S Williams, Christopher J K Bulstrode: Bailey and Love's Short practice of Surgery 24th edition 2004.p.17-28.
4. IDF Diabetes Atlas (6 ed.) : International Diabetes Federation. 2013. ISBN 2930229853. p. 7.
5. David G. Gardner.Insulin Basics, American Diabetes Association: Retrieved 24 April 2014.
6. Shah VN, Moser EG, Blau A, Dhingra M, Garg SK. The future of basal insulin. Diabetes Technol Ther. 2013;15:727–32. [PubMed: 23965036]
7. Teresa L. Pearson, M.S., R.N., CDE Practical Aspects of Insulin Pen Devices Journal of Diabetes Science and Technology Volume 4, Issue 3, May 2010
8. Nauck M. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care. 2013;36:2126–2132.,
Asmar M, Holst JJ.Glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide: new advances. Curr Opin EndocrinolDiabetes Obes. 2010;17:57–62.
Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1[7–36 amide] but not of the synthetic human gastric inhibitory polypeptide in patients with type 2 diabetes mellitus. J Clin Invest. 1993;91:301–307.
9. Barrington P, Chien JY, Tibaldi F, Showalter HD, Schneck K, Ellis B. LY2189265, a long-acting glucagon-like peptide-1 analogue, showed a dose-dependent effect on insulin secretion in healthy subjects.Diabetes Obes Metab. 2011;13:434–438.
Glaesner W, Vick AM, Millican R, et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue doi: 10.1210/en.2014-1722 press.endocrine.org/journal/endo 2427 Downloaded from https://academic.oup.com/endo/article-abstract/156/7/2417/2422864
by guest on 07 January 2018 LY2189265, a Fc fusion protein. Diabetes Metab Res Rev. 2010;
26:287–296.
Barrington P, Chien JY, Showalter HD, et al. A 5-week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long-acting glucagon-like peptide-1 analogue, in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:426–433.
Vahle J, Byrd R, Blackbourne J, et al. Effects of dulaglutide on thyroid C-cells and serum calcitonin in male monkeys. Endocrinology. 2015;156(7):2409–2416.
10. Rima B. Shah, Manhar Patel, David M. Maahs, and Viral N. Shah, Insulin delivery methods: Past, present and future International Journal of Pharmaceutical Investigation.
11. Michele Pisano, PharmD, CGP, Overview of Insulin and Non-Insulin Delivery Devices In the Treatment of Diabetes December 2014 • Vol. 39 No. 12.
12. Douglas A. Kerr. Insulin Pens and Their Mechanisms Issue 5 November 30, 2015
13. Nadkarni P, Chepurny OG, Holz GG: Regulation of glucose homeostasis by GLP-1. Prog Mol Biol Transl Sci. 2014;121:23-65. doi: 10.1016/B978-0-12-800101-1.00002-8. [PubMed:24373234]Sanford M: Dulaglutide: first global approval. Drugs. 2014 Nov;74(17):2097-103. doi: 10.1007/s40265-014-0320-7. [PubMed:25367716]
http://dx.doi.org/10.1080/00325481.2016.1218260
14.John E. Anderson, Vivian T. Thieub, Kristina S. Boyce, Ryan T. Hietpasd and Luis-Emilio Garcia-Perezb. Dulaglutide in the treatment of adult type 2 diabetes: a perspective for primary care providers postgraduate medicine, 2016 vol. 128, no. 8, 810–821.
15.Z. Skrivanek1, B. L. Gaydos1, J. Y. Chien1, M. J. Geiger2, M. A. Heathman1, S. Berry3, J. H. Anderson4, T. Forst5, Z. Milicevic1 & D. Berry3 Dose-finding results in an adaptive, seamless, randomized trial of once-weekly dulaglutide combined with metformin
in type 2 diabetes patients (AWARD-5) Lilly Diabetes, Eli Lilly and Company, Indianapolis, IN, USADiabetes, Obesity and Metabolism 16: 748–756, 2014.
16. Masanori Emoto1), Yasuo Terauchi2), Akichika Ozeki3), Tomonori Oura3), Masakazu Takeuchi, et al1114A 1-year safety study of dulaglutide in Japanese patients with type 2 diabetes on a single oral hypoglycemic agent: an open-label, nonrandomized, phase 3 trial Endocrine Journal 2015, 62 (12), 1101-111.
17. Glenn Matfin, MD, MSc, ChB, FFPM, FACE, FRCP,1 Kate VanBrunt, BA,2 Alan G. Zimmermann, PhD,3 Rebecca Threlkeld,MS, RDN,3 and Debra A. Ignaut, BS, RN, CDE, CDTC3 Safe and Effective Use of the once-weekly Dulaglutide Single-Dose Penin Injection-Naïve Patients WithType 2 DiabetesJournal of Diabetes Science and Technology2015, Vol. 9(5) 1071–1079© 2015 Diabetes Technology Society
18. Richard A. Byrd, Steven D. Sorden, Thomas Ryan, Thomas Pienkowski,* Richard LaRock, Ricardo Quander, John A. Wijsman, Holly W. Smith,*Jamie L. Blackbourne, Thomas J. Rosol, Gerald G. Long, Jennifer A. Martin,and John L. Vahle, Chronic Toxicity and Carcinogenicity Studies of the Long-Acting GLP-1 Receptor Agonist Dulaglutide in Rodents Department of Toxicology, Pathology, and Drug Disposition (R.A.B., J.A.W., H.W.S.,J.L.B., J.A.M.,
J.L.V.), Eli Lilly and Company, Indianapolis, Indiana 46285;



No comments:
Post a Comment
We are happy that you want to comment, please note that your comment will be reviewed first before it is published.
If you like the article! You can share it with your friends and colleagues by pressing at social media buttons provided to the left of the page.
NO word verification or sign up is required!